A 3D-printable ink can change color with electricity, letting people make pixel displays, soft robots, and 3D electronic devices.
Creating complex, 3D structures that are electrically conductive and can change color has been a major challenge for optoelectronic applications. Traditional 3D printing methods could not produce materials that combine conductivity with electrochemical switchability, limiting their use in pixel displays, soft robotic actuators, and other devices that require dynamic, controllable materials.
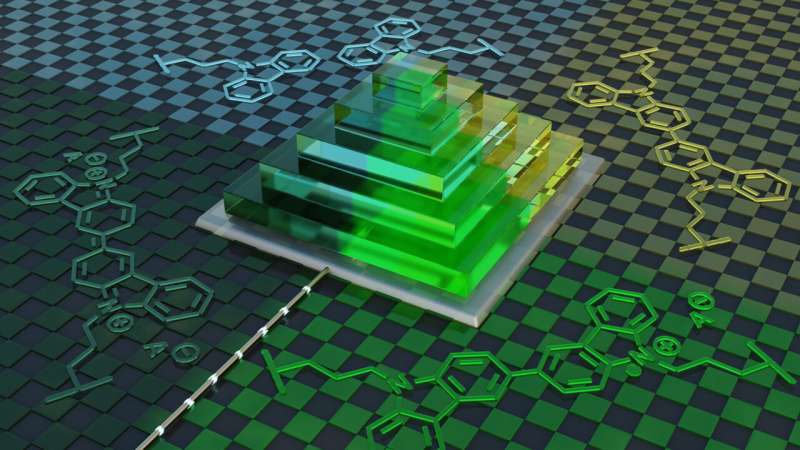
Researchers at the universities of Heidelberg and Stuttgart addressed this problem by developing a new methacrylate-based “ink” containing redox-active carbazole groups. These redox units allow the polymer chains to donate or accept electrons, making the material both electrically conductive and capable of reversible color changes under electrochemical stimulation. Structures printed with this ink remain electrochemically switchable even after fabrication, with pixel-level control—including in three-dimensional architectures.
The ink is compatible with digital light processing (DLP), a high-resolution, light-based 3D-printing method where UV light selectively solidifies a light-sensitive “ink” layer by layer. Using DLP, the researchers fabricated two-dimensional pixel arrays, checkerboard patterns, and multi-layered pyramids. Initially almost transparent, these structures could be switched from light green to dark green and nearly black through electrochemical stimulation, with the color change fully reversible and controllable in the third dimension, including the height of the structures.
By combining high-resolution light-based 3D printing with electrochemically active redox polymers, this approach creates new opportunities for additive manufacturing of 3D-printed optoelectronic devices, pixel displays, and soft robotic actuators with switchable volume or color. The work was carried out at the Institute for Molecular Systems Engineering and Advanced Materials, in close collaboration with experts in conducting polymers and electrochemical switching at the Institute of Polymer Chemistry.